“
Sublimation is a unique process in which water transitions directly from a solid (ice) to a gas (vapor) without passing through the liquid state. This fascinating phase change is essential to the water cycle, contributing to natural phenomena such as the sublimation of snow and ice in cold regions. In this blog, we'll explore 20 interesting facts about sublimation, focusing on its role in the states of water and its importance in nature. 1
1
1
1
”
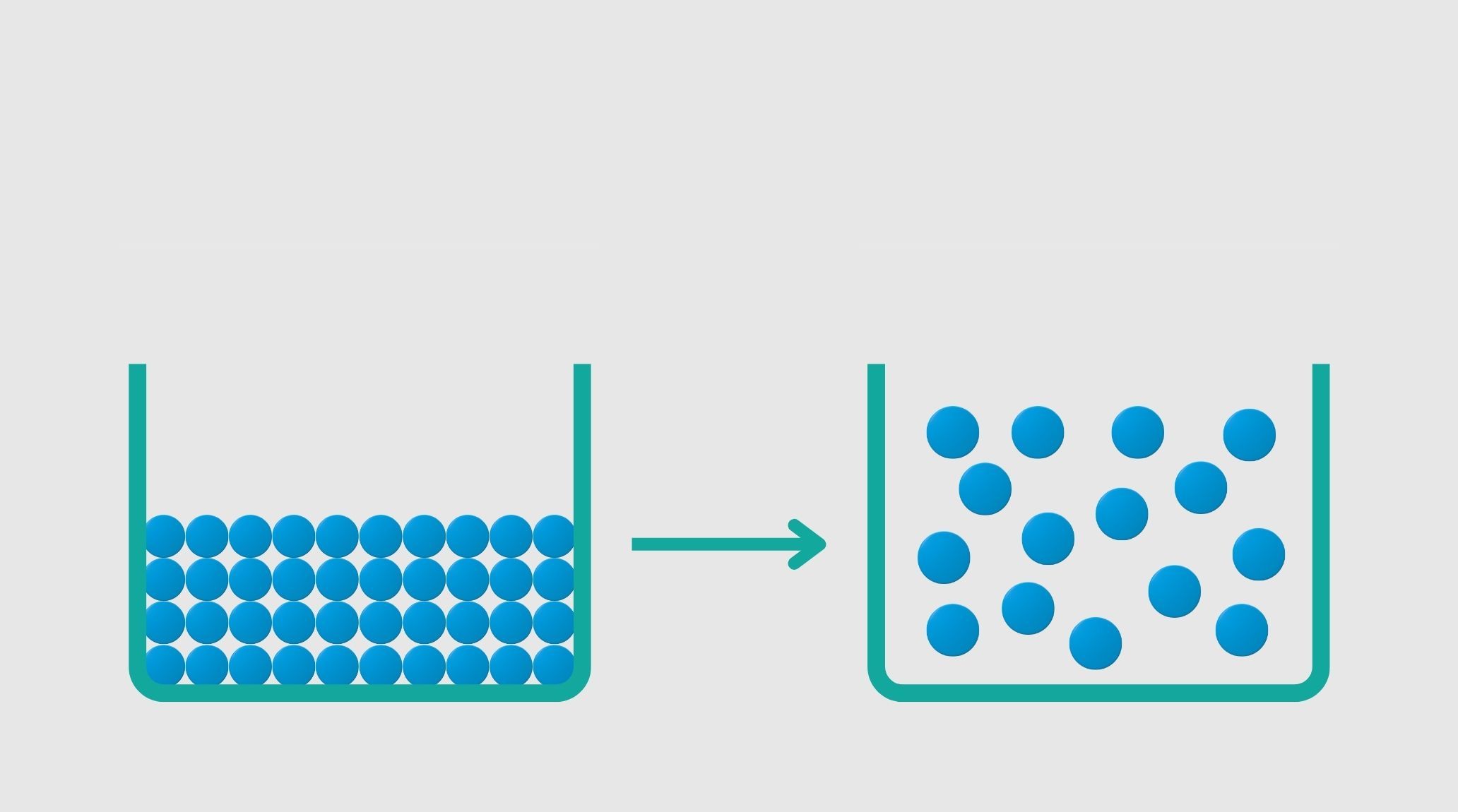
Sublimation is the transition of a substance directly from a solid to a gas without passing through the liquid phase. This phenomenon occurs under specific conditions of temperature and pressure. 1
Sublimation is used in freeze-drying, where frozen food or pharmaceuticals undergo low pressure, allowing ice to sublimate. This preserves the structure and extends shelf life without refrigeration. 2
Iodine crystals exhibit sublimation at room temperature, releasing a violet gas. This property is harnessed in laboratories for purifying iodine and studying its properties, as the gaseous iodine can be collected and condensed back into solid form. 3

Sublimation is also observed in snow and ice on a sunny winter day. Even when the temperature is below freezing, the snow can slowly disappear directly into water vapor due to the sublimation process without first melting into liquid water.
In the sublimation process, the energy required to break the solid bonds and enter the gaseous phase is known as the sublimation energy. This energy is different from the heat of fusion, which is required for melting a solid into a liquid. 4
The principle of sublimation is used in certain industrial processes, such as creating high-purity materials. For instance, metals like zinc can be purified using sublimation, where the metal vapor is collected and solidified, removing impurities.5
Sublimation naturally occurs in polar regions, where ice and snow turn directly into vapor due to low pressure and sunlight, influencing the unique weather patterns in the Antarctic and Arctic. 6
Sublimation is essential in the production of certain types of pigments and dyes. For example, sublimation dyes are used in printing to create vibrant, long-lasting images on fabrics and other materials by turning solid dye directly into gas. 7
Sublimation plays a key role in planetary atmospheres. As comets near the sun, their ices sublimate, releasing gases that form the comet’s visible coma and tail, creating its dynamic appearance. 8

Dry ice, made of solid carbon dioxide, sublimates at temperatures above -78.5°C (-109.3°F). This unique property is used in special effects for fog and smoke without leaving any residue, making it ideal for theatrical and entertainment applications.
In chemistry, sublimation separates substances with varying sublimation points, enabling chemists to purify compounds and analyze properties by capturing and studying them in their pure gaseous form. 9
In material science, sublimation is used to coat and create thin films. Metal and polymer films can be deposited onto surfaces through sublimation techniques, yielding uniform coatings with specific properties for industrial applications. 10
The sublimation of water ice is a significant process in glaciology. It contributes to the mass loss of glaciers and ice caps, affecting sea levels and climate patterns, as the sublimated ice vapor eventually precipitates elsewhere in the environment. 11
Some solids, like ammonium chloride and camphor, sublimate at room temperature, making them useful in educational demonstrations to illustrate the solid-to-gas transformation for students. 12
Sublimation is used in creating high-tech materials. In semiconductor production, thin layers are deposited onto surfaces, enabling the development of intricate electronic components essential for modern technology. 13
Sublimation is crucial for studying planetary atmospheres. It helps scientists understand the composition and behavior of celestial bodies, like Jupiter’s and Saturn’s icy moons, which experience sublimation due to temperature changes. 14
In physics, sublimation offers insights into the kinetic theory of matter. Studying how particles transition from solid to gas helps researchers understand molecular interactions and energy dynamics in phase changes. 15
Sublimation is employed in specialized cleaning processes. Freeze-drying, removes water from delicate materials like food and pharmaceuticals, preserving quality while reducing the need for harsh chemical cleaners. 16
Sublimation is observed in everyday phenomena like the disappearance of mothballs. These small, solid spheres slowly turn into gas, releasing their odor and helping to repel insects, all without leaving behind any liquid or solid residue. 17
Sublimation plays a key role in sublimation-driven geysers on Mars. These geysers expel water vapor and gases as ice sublimates beneath the surface, influencing the planet's atmospheric phenomena and surface features. 18


