“
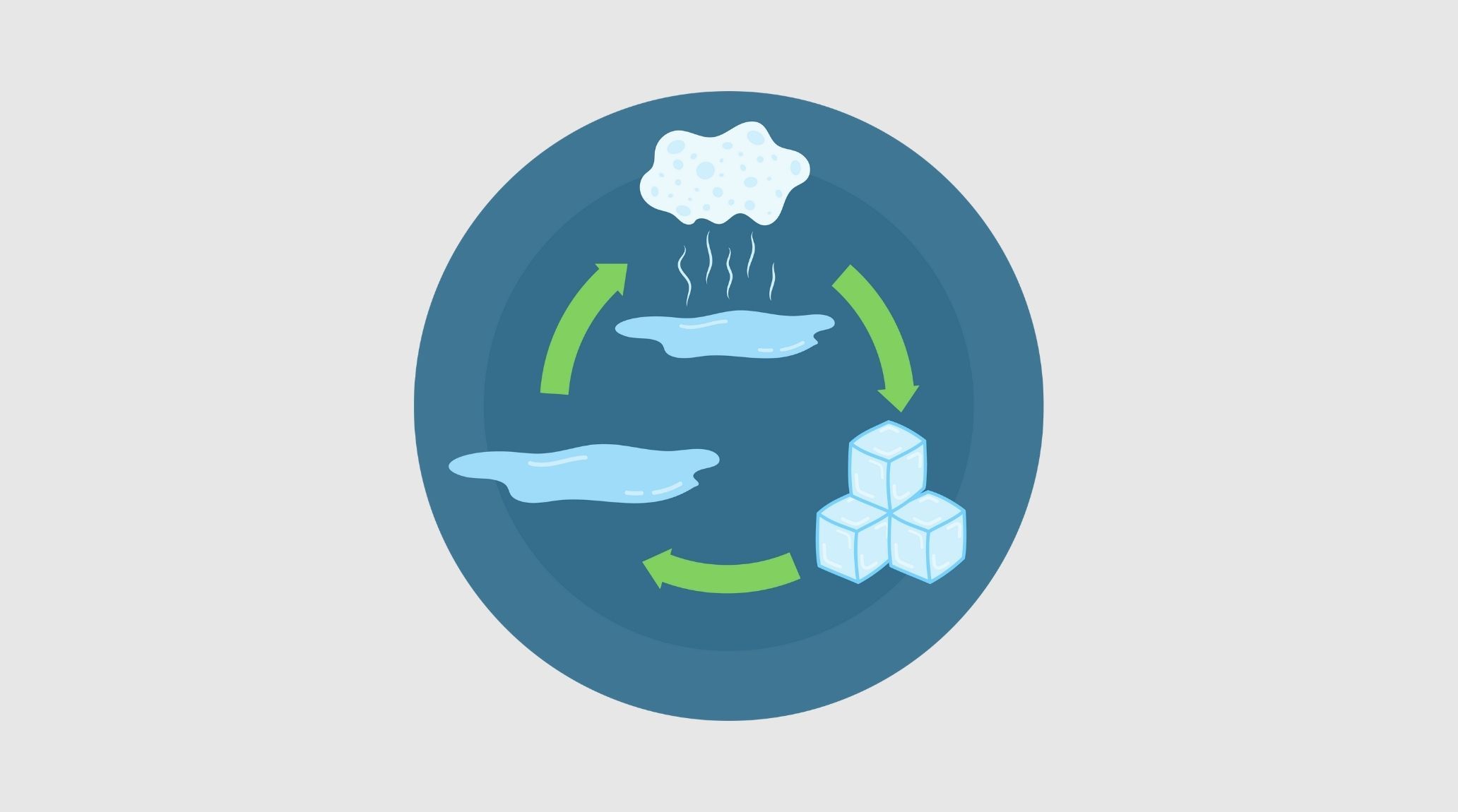
Water exists in three different states: solid, liquid, and gas. These states are essential to life on Earth and form the basis of many natural processes. In this blog, we will explore 20 interesting facts about the states of water, shedding light on how water transitions between these states and their importance in our daily lives. 1
1
1
1
”
Solids maintain a fixed shape and volume because their particles are closely packed in a regular pattern. This tight arrangement means solids can withstand forces without changing shape, such as ice remaining firm even in warm water. 1
Liquids have a definite volume but no fixed shape, adapting to the container they’re in. The particles in liquids are less tightly packed than in solids, allowing them to flow and take the shape of their container. 2
Gases lack both a fixed shape and volume, expanding to fill their container. The particles in gases are far apart and move rapidly, which explains why a balloon inflates and gas diffuses throughout the space. 3
Solids are rigid and resist deformation because their particles vibrate in place rather than moving freely. This rigidity is due to strong intermolecular forces that hold the particles together, like in the structure of a diamond or steel. 4
Liquids can flow and conform to the shape of their container because their particles can slide past each other while remaining close. This fluidity allows liquids to be poured, like water or oil, adapting to different vessel shapes. 5
Gases spread out to occupy any available space because their particles move rapidly and are far apart. This explains why a single puff of air can quickly fill an entire room, diffusing evenly throughout the available volume. 6

Liquids have surface tension, which is caused by cohesive forces among their molecules. This effect allows small objects to float on the surface of a liquid if they don’t break through, like water striders walking on water.
Gases are compressible because the space between their particles is large. This means that gases can be squeezed into smaller volumes, such as when air is pumped into a bicycle tire, reducing its volume while increasing pressure. 7
Solids can transfer heat through conduction because their particles are tightly packed, allowing them to pass energy efficiently. For instance, a metal spoon heats up quickly when placed in a hot cup of coffee due to this efficient energy transfer. 8
Liquids can exhibit viscosity, which is a measure of their resistance to flow. High-viscosity liquids, like honey, flow slowly due to stronger intermolecular forces, while low-viscosity liquids, like water, flow easily due to weaker forces. 9
Gases mix uniformly with other gases due to their high kinetic energy and random motion. This property explains why the smell of perfume spreads quickly throughout a room as gas particles diffuse and mix with air molecules efficiently. 10
Solids vary in hardness based on their molecular structure. Diamonds are extremely hard due to strong covalent bonds, while chalk is much softer because its molecular bonds are weaker, making it more prone to breakage. 11
Liquids can evaporate at temperatures below their boiling point. This occurs when surface molecules gain enough energy to escape into the air, explaining why water puddles gradually disappear on sunny days through evaporation. 12
Gases exert pressure on their containers due to the collisions of their particles with the container walls. This pressure increases with temperature and decreases with volume, explained by the ideal gas law: PV=nRT. 13
Solids can deform under stress but return to their original shape once the stress is removed. This elasticity is seen in rubber bands, which stretch when pulled but return to their original size when the force is released. 14

Gases can dissolve in liquids to form solutions, such as carbon dioxide in soda. The solubility of gases in liquids depends on temperature and pressure, with higher pressure and lower temperatures generally increasing gas solubility.
Solids can be crystalline or amorphous. Crystalline solids, like quartz, have a well-ordered structure, while amorphous solids, like glass, have a more random arrangement of particles, affecting their properties and behaviors. 15
Liquids can be compressed slightly, though not as much as gases. This slight compressibility is due to the small amount of space between their molecules, which can be reduced under high pressure, affecting liquid density and volume. 16
Gases expand when temperatures increase because their particles move faster. This explains why hot air balloons rise, as the heated air inside expands, becoming less dense than the cooler surrounding air, allowing the balloon to float. 17
Some solids, like dry ice (solid CO2), can undergo sublimation, directly changing from a solid to a gas without passing through the liquid phase. At room temperature, dry ice sublimates, creating a visible vapor without melting. 18


